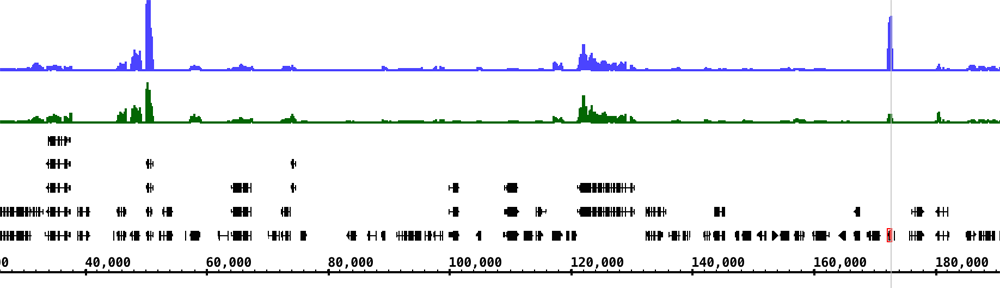
Our building’s basement contains several temperature-controlled rooms where we grow plants under controlled conditions. We set up one of these rooms for growing rice. Here, we explain how we did it in hopes this will help other groups grow rice for research.
The protocol described here is based on this protocol from Purdue University. [link: “this protocol” to the Web page at Purdue. Don’t mention it again after this.]
Materials
- seeds
- container
- soil
- pea gravel
- fertilizer
- lighting
Rice seeds. We got our received seeds (wild type Nipponbare) from the Kieber lab at UNC Chapel Hill.
Soil. We use 50/50 mix of profile greens and Promix soil. Profile Greens: profile is the brand, greens is the grade. Its intended for landscaping idn golf courses and we bought our supply through a golf course supply store in south Charlotte. These fine clay granules look like sand but wick up water (much like the clay granules used in cat liter do) and create an evenly hydrated medium. The Promix soil is a common garden mix, we ordered it online.
Container. Plastic tub – 2ft x 3ft x 8in deep.
Pea gravel. [Explain where this comes from] We use pea gravel to help with controlling gnats.
Fertilizer. Scotts 15-15-15 fertilizer. This comes as a solid mixture to be dissolved in water. I mix 133g of it in 1L of water to make a concentration that is 100x. I dilute this in a 2L pitcher to 1x and apply 6L up to three times per week.
Lighting. We use the lumigrow XXXX, which uses blue, red, white LEDs. The red and blue LEDs emit only the wavelengths of light that are most efficiently utilized by plants. LEDs are more efficient than other types of bulbs in terms of the percentage of energy that is lost to heat, and the amount of light generated per unit of energy. These fixtures take it one step further by being more efficient in terms of light used by the plant per light emitted by the fixture. The white LEDs are primarily for my benefit; the red-blue is easier on the eyes with a little white mixed in, and there is a little switch so I can have just white light if I need to do anything in the growth chamber. The lights are plugged into a timer so they are only on for 14 hours each day.
Methods
- Prepare soil. Explain what to do.
- Set up shelving. Explain what to do.
- Set up lighting. Explain what to do.
- and etc.
Heat and humidity. Our tub of rice and LED lights are set up in a growth chamber in the basement. The chamber is temperature controlled (we have it set to 28C) and humidity controlled (we have set to about 50% RH).
Diary
19 Sept. 2014 – Planted Rice seeds
Used the seeds from an envelope labelled: Nipponbare wild type, collected 07/15/13, envelope 6 of 6, see page 58 notebook 36.I tried to get seeds from several different panicles and I tried to discard seeds that seemed skinny. I set up one fo the ikea tubs, ~50%/50% mix of profile greens grade clay grandules and Pro mix. I wetted the soil mixture and mixed it. The seeds are in a grid of 5 rows x 6 columns with 2 seeds per spot about an inch or two apart. I marked each seed spot with a little green wire.
I put the 32 seeds that I didn’t use into a plastic dis with water and left on top of the soil. I covered teh top of the tub with clear-cling plastic wrap so the seeds would not get dry over the weekend.
22 Sept. 2014
Took off the plastic wrap. 29 of the 32 seeds in teh dish have germinated. They are all at the bottom of the dish. The three seeds that did not germinate are skinny and floating. Not many (hardly any) plants poking out of the soil yet.
30 Sept 2014
100% of the seeds have germinated. Some have the first leaf blade.
2 October 2014
I bought pea gravel at Lowes hardware, and added it to the surface of the the soil around the rice plants.
3 October 2014
Watered + fertilized the rice. I still have the 100x concentrate of liquide 15-15-15 Scotts fertilizer from the last time we grew rice. I gave the tub 4L of 1x and then hose water, and left it with an inch of water over the pea gravel.
6 October 2014
The pea gravel is still wet, and a bit green. Next time I guess I should just water it up to the stones, not above them.
10 October 2014
Added some fertilizer to the soil (3L of 1x) and hose water.
I removed 9 plants to reduce crowding (when I seeded them I didn’t actually expect all of them to germinate). Took samples on liquid nitrogen (see page 123, notebook 40 for more info).
I have a small digital thermometer in the chamber. It reads 86F if it is shaded by the rice and 90F if not shaded. It records the min/max over 24 hours, and I think the temperature difference is in sync with the light cycle (7am to 9pm).
14 October 2014
Mason and I moved the tub of rice from the shelf to the floor. We have two lumigrow light fixtures on one shelving unit. One fixture is suspended from the top and illuminates the shelf below. The other is suspended from the middle shelf and illuminates the floor where the tub of rice is.
Something a little odd, the plants at the edge of the tub seem to be the healthiest–tallest greenest leaves. Are the ones in the middle drying or maybe burning because they are too close to the light? –if that’s the case, the move should help, they have more space now.
I added 4L of 1x fertilizer to the tub of rice. Then I added some hose water–partially just to rinse any fertilizer of of the leaves.

25 days after the initial seeding. The tub was moved to the floor to have more space between the tub and the light fixture, and to make space for the next batch of seedlings (top shelf).
15 October 2014 – pictures

26 days after initial seeding.

26 days have initial seeding, plants are tillering.
17 October 2014
Watered with 6L of 1x, then hose water.
20 October 2014
Used the hose to add more water to the tub of rice. The plants look good, the rocks were dry, and the soil was not saturated even at teh bottom. I filled it until the water was around the rocks. I think things dry out much faster in here when I have two lights on, and the rice must pull more water from the soil now that they are larger.
22 October 2014
Added 8L of 1x fertilizer to the tub of rice. Then added water from the hose until there was water around the bottoms of the rocks. This is the same aproximate water level that I reached on Monday (2 days ago) which means that the tub looses more than 8L in 2 days (or >4L/day).
24 October 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
27 October 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
I showed Mason how to do this. : )
29 October 2014
Mason watered the rice: Added 6L of 1x, then added hose water to fill to the top of the tub.
****skulls and daggers! I turned on the overhead light when I came in on Monday and I forgot to turn it off. The overhead lights are fine except that they are not on a timer. During the rice day (when the LEDs are on) its hard to tell if the overhead lights are on, but during the rice night, the overhead lights will stay on. I’ve heard that Nipponbare is very sensitive to light cycle, and it will take forever for it it to go to seed if the days are too long. I hope this does mess things up. : ( These lights are not nearly as bright as the LEDs, hopefully it’ll have only as much affect as moonlight or starlight…two nights of artificial full moon to go with the artificial sun light.
31 October 2014
I wanted to use 100% fertilizer water to fill the tub today, but I got up to 12L and that didn’t even bring it up to the level of the rocks…The soil was really dry, but the plants look fine, so it must not have been dry for long. I used hose water to fill it up the rest of the way to the top of the tub.
4 November 2014
Made a new bottle of 100x fertilizer from the solid stuff. See details in Mason’s notebook: page 78 notebook 58. For full original protocol and rationale, see my notebook: page 78 notebook 36.
5 November 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
7 November 2014
Added 6L of 1.5x, then added hose water to fill to the top of the tub. (I thought the leaves looked like an overly light green, which made me think that they weren’t getting enough N).
The rice grew up into the fan of the LED light. So April and Nowlan helped me move the shelf up. The rice is on the floor, so the this moves the light further away. (Moved it 10 inches).
10 November 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
12 November 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
14 November 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
17 November 2014
Added 6L of 1x, then added hose water to fill to the top of the tub.
Nowlan and I cut up one of the plants and from the tub to see if it had entered the “booting” stage, when the panicle begins to develop.

One plant from the tub.
We cut off individual tillers….

One tiller.
The largest tiller on the plant we cut has at least 5 distinct “crowns”.

59 days after initial seeding. Cross section of the largest tiller on the plant we cut up.
Compare this to these reference images:

Reference images showing the green ring that becomes visible with the initiation of panicle development.
Figure image from “Chapter 2: Rice Growth and Development” by Karen Moldenhauer, Charles E. Wilson, Jr., Paul Counce and Jarrod Hardke. Photo from “Understanding of Growth Stages is Critical in Rice Production” from the LSU college of agriculture.
We looked at several tillers from this plant, the others had fewer crowns: 3, 5, 2 and 4.

Smaller tiller from the same plant from the tub.
This range of stages may be very convenient when we collect samples for our time course. Cutting up one plant might give us several sample options.
We also looked at one of the plants that was removed from the tub on October 10th. I set a few of them aside to grown in a window sill in one of the offices. They are much less developed than their counterparts downstairs (due to getting only window light in November, lower temperature, lower humidity, much less fertilizer, general neglect–its a harsh life).

Window sill plant.

Cross section of a tiller from the window sill plant.
18 November 2014
The leaves are starting to get into the fan again. >: [
That means they grew nearly 10 inches in 11 days.
I used a pair of scissors to essentially mow the leaves that were too close to the light (cut about 1 to 6inches per leaf).
The plants in the middle of the tub (directly under the light) are a bit taller than the ones on the edge, but only by a few inches. At least they were, before the mowing.